Meniscal Regeneration By Meniscal Scaffold Implant Actifit Polyurethane Scaffold
Meniscal Regeneration By Meniscal Scaffold Implant Actifit Polyurethane Scaffold
article written/reviewed by doctors of Asia Medical Specialists
(Last updated on: Oct 13th 2020)
Introduction
Irreparable major meniscal tears pose a very difficult problem in the young and active patients who sustain these kinds of injuries. Arthroscopic partial meniscectomy (Fig. 1), the most commonly used treatment option for meniscus tear creates substantial number of patients suffering the effect of a lost meniscus cartilage which is knee pain and possible degenerative joint disease in long term. It is extremely important to preserve the meniscus as much as possible to avoid degenerative knee joint progression.
|
The goal of the meniscal regeneration is to restore the load-bearing function of the meniscus, decrease symptoms and provide chondroprotective (articular cartilage protection) effects.
Increased awareness of potentially detrimental outcomes following partial meniscectomy led to the development of a novel meniscal scaffold, Actifit™, by Orteq Bioengineering (London) (Fig. 2). It received the CE Mark in July 2008 for the treatment of medial or lateral irreparable partial meniscal tears. Actifit™ consists of highly interconnected porous synthetic material enabling tissue ingrowth. Over time, transformation into meniscus-like tissue takes place as the implant slowly degrades. Furthermore, Actifit™ is made of an aliphatic polyurethane, which provides optimal mechanical strength, biocompatibility, porosity, safe degradation, and ease of use
required for the indication [1].
 Fig. 2 Actifit polyurethane scaffold implant |
Meniscal scaffold implants support the in-growth of new "meniscus like" tissue with the aim of alleviating postmeniscectomy knee pain and preventing further articular cartilage degeneration [2]. The Actifit polyurethane scaffold has been shown to be a safe, effective implant, for the treatment of patients with pain as a result of segmental medial meniscus loss at 2 years [3].
What does the procedure involve?
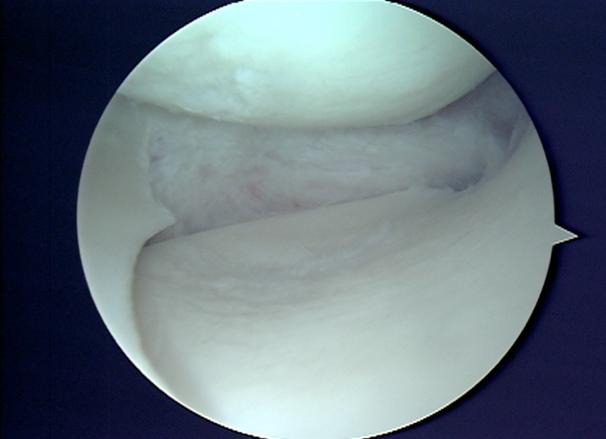
Under general or regional anaesthesia, surgeon will use the joint camera (arthroscopy) to examine the knee thoroughly through two or three keyholes on your knee. Then we start preparing the implant site, clean up the meniscus defect (Fig. 3). The remaining meniscus rim should be kept intact. We provide extra blood supply by making puncture holes in the peripheral rim. We also trimmed in a square shape the anterior and the posterior attachment points to accept the scaffold. Once the implant site is prepared, we measure the meniscus defect using a specifically designed measuring device (Fig. 4). The scaffold is removed from the sterile packaging, is measured and trimmed to fill the defect (Fig. 5). Then the implant is inserted into the defect. For the scaffold fixation, we suture the implant to the host meniscus with all inside technique (Ultra-fast fix, Smith & Nephew) (Fig. 6). Finally, we check the stability of the implanted scaffold. The wound is closed (only skin stitches), and the knee is placed in compressive dressing. A knee brace is applied.
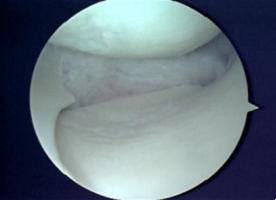 Fig. 3 Clean up the meniscus defect |
 Fig. 4 Measure the size |
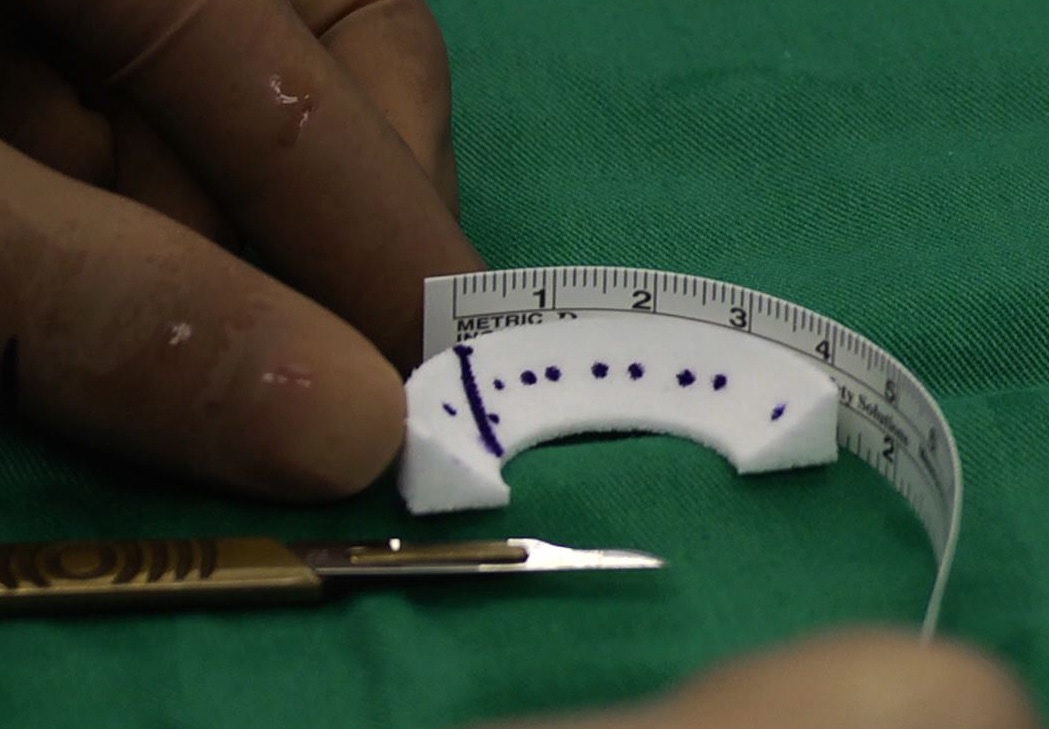 Fig. 5 Prepare the implant |
.jpg?1470295691017) Fig. 6 Suture the implant |
What are the benefits I might get?
Meniscal scaffold implants support the in-growth of new "meniscus like" tissue with the aim of alleviating postmeniscectomy
knee pain and preventing further articular cartilage degeneration. It thus improves the function of your knee [4] and maintains the active lifestyle.
How good are my chances of getting those benefits?
The Actifit polyurethane scaffold has been shown to be a safe, effective implant, for the treatment of patients with pain as a result of segmental medial meniscus loss at 2 years [3, 4].
Are there alternative procedures?
No clinically proven alternative procedure for the partial loss of meniscus.
What are the risks of the procedure [3, 4, 5]?
1. any complication associated with arthroscopy including, but not limited to, damage to articular cartilage, healthy meniscus tissue, neurovascular structures of the knee.
2. restricted freedom of movement and/or pain after rehabilitation period.
3. any complication associated with general surgery. (blood loss, deep vein thrombosis, infection, pulmonary embolism, etc.)
4. any negative effects associated with drugs or ancillary devices used during implantation.
What care will I need after the procedure?
Physiotherapy starts from the first postoperative day. Knee brace is applied. Weight bearing and the range of motion on the knee then increase progressively.
• Range of motion (bracing): Week 1-2: 0-30°; Week 3: 0-60°; Week 4-5: 0-90°; Week 6: 90° and more
• Walking: Week 1-3: non weight bearing; Week 4-8: progressive loading (10kg per week for patients weighing ≥60kg; 15kg per week for patients weighing ≥90kg); Week 9: full weight bearing
What may happen if I don't have the procedure?
Accept the partial loss of the meniscus. Accept the knee pain associated with the partial loss of the meniscus and the possible degenerative joint disease in long term.
Reference
1. Verdonk PCM, Van Laer MEE, Verdonk R. Meniscus replacement: from allograft to tissue engineering. Sports Orthop Traumatol
2008;24(2):78–82.
2. Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs E. Tissue in-growth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med 2011;39(4):774–82
3. Peter Verdonk,*y MD, PhD, Philippe Beaufils,z MD, Johan Bellemans,§ MD, PhD, Patrick Djian,|| MD, Eva-Lisa Heinrichs,{ MD, PhD,
Wouter Huysse,# MD, Heinz Laprell, MD, Rainer Siebold,yy MD, PhD, Rene´ Verdonk,y MD, PhD, and the Actifit Study Groupzz.
Successful Treatment of Painful Irreparable Partial Meniscal Defects With a Polyurethane Scaffold -Two-Year Safety and Clinical
Outcomes. American Journal of Sports Medicine, published on February 9, 2012 doi:10.1177/0363546511433032
4. Turgay Efe , Alan Getgood, Markus D. Schofer, Susanne Fuchs-Winkelmann, Dieter Mann, Ju¨rgen R. J. Paletta, Thomas J. Heyse. The
safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency.
Knee Surg Sports Traumatol Arthrosc (2011) DOI 10.1007/s00167-011-1779-3.
5. Spencer SJ, et al. Meniscal scaffolds: Early experience and review of the literature. Knee (2012), doi:10.1016/ j.knee.2012.01.006
| Copyright ©2017 Asia Medical Specialists Limited. All rights reserved. |